Photochemical Reaction in Solid State
Photochemical Crystals
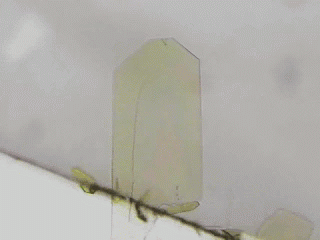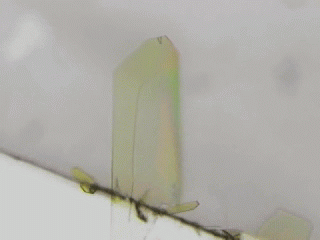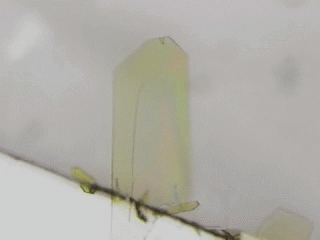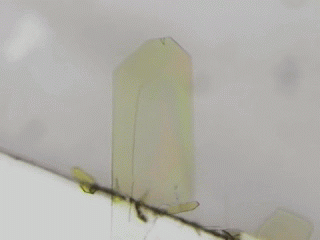 Crystal is an organization of molecules arranged periodically in three dimensions. Crystals have been thought to be rigid and fragile. However, in 2007, bending motions of diarylethene crystals upon UV light irradiation were reported by Irie and his coworkers [1]. This phenomenon is called as photomechanical effect. The photomechanical bending motion is originated from crystal structural change due to molecular isomerization upon light irradiation. Our group has reported several photomechanical crystals such as azobenzene [2,3], salicylideneaniline [4], furylfulgide [5]. Because photomechanical crystals can convert light energy into mechanical work directly, photomechanical crystals will be useful to next-generation actuators. We aim to discover new photomechanical crystals and develop further functions. Now, we are working on azobenzene, anthracene, HABI, and PABI crystals.
Crystal is an organization of molecules arranged periodically in three dimensions. Crystals have been thought to be rigid and fragile. However, in 2007, bending motions of diarylethene crystals upon UV light irradiation were reported by Irie and his coworkers [1]. This phenomenon is called as photomechanical effect. The photomechanical bending motion is originated from crystal structural change due to molecular isomerization upon light irradiation. Our group has reported several photomechanical crystals such as azobenzene [2,3], salicylideneaniline [4], furylfulgide [5]. Because photomechanical crystals can convert light energy into mechanical work directly, photomechanical crystals will be useful to next-generation actuators. We aim to discover new photomechanical crystals and develop further functions. Now, we are working on azobenzene, anthracene, HABI, and PABI crystals.
Topochemical Polymerization
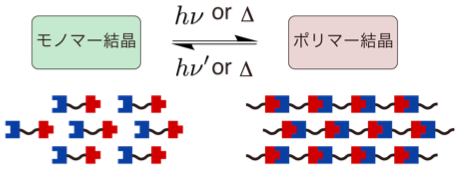 Organic molecules able to polymerize and depolymerize are expected as renewable polymer materials. Topochemical reaction undergoes smoothly in crystal keeping its crystallinity. Therefore, organic solvents are not required in the reaction unlike ordinary chemical reactions. This means topochemical polymerization will probably contribute to effective utilization of fossil resources and to development of low-carbon societies. We aim to design molecules which can occur reversible topochemical polymerization and to analyze its mechanism.
Organic molecules able to polymerize and depolymerize are expected as renewable polymer materials. Topochemical reaction undergoes smoothly in crystal keeping its crystallinity. Therefore, organic solvents are not required in the reaction unlike ordinary chemical reactions. This means topochemical polymerization will probably contribute to effective utilization of fossil resources and to development of low-carbon societies. We aim to design molecules which can occur reversible topochemical polymerization and to analyze its mechanism.
Microwave Chemistry
Since microwave oven was industrialized in 1950s, microwave has been familiar to us and changed our dairy life. Microwave has made cooking easier and faster. The heating mechanism is that dipoles are oscillated by microwave and then molecules generate frictional heat. In addition, non-heating effect has attracted attention as "microwave effect" recently. Some papers have reports that microwave-assisted synthesis reacted faster and reached higher yields than conventional heating synthesis in oil bath. However, the mechanism of promoting reactions by microwave is still unclear. Therefore, we aim to elucidate microwave effect. Now, we are working on microwave effect in ester reactions.
References
[1] S. Kobatake, S. Takami, H. Muto, T. Ishikawa, M. Irie, Nature, 446, 778-781 (2007).
[2] H. Koshima, N. Ojima, H. Uchimoto, J. Am. Chem. Soc., 131, 6890-6891 (2009).
[3] H. Koshima, N. Ojima, Dyes and Pigments., 92, 798-801(2012).
[4] H. Koshima, K. Takechi, H. Uchimoto, M. Shiro, D. Hashizume, Chem. Commun., 47, 11423-11425 (2011).
[5] H. Koshima, H. Nakaya, H. Uchimoto, N. Ojima, Chem. Lett., 41, 107-109 (2012).
[6] P. Johnston, C. Braybrook, K. Saito, Chem. Sci., 3, 2301 (2012).
