Physicochemical analysis of chiral pharmaceutical molecular behavior in solution and solid state
Chiral molecules are widely used in the multiple fields including food science, agriculture and medicinal drug as highly functionalized molecules. Chirality in medicinal drug, especially, is of great importance because it enhances the binding affinity of drug with its receptor and yields higher drug efficacy. We are focusing on thalidomide and its derivatives which recently attract worldwide attentions again.
Thalidomide was developed at a German pharmaceutical company in 1957 as a safe sedative hypnotic. In those days many pregnant women took thalidomide to prevent morning sickness, but their baby suffered from severe birth defects (phocomelia). In 1962 a German pediatrician Widukind Lenz first pointed out the relationship between thalidomide and teratogenesis [1]. The “thalidomide syndrome” exerted a big impact on the world and then thalidomide was withdrawn soon from the worldwide market.
In subsequent work, the relationship between teratogenicity and chirality in thalidomide was reported. The study using animal examination revealed that only (S)-isomer of thalidomide was a potent teratogen whereas opposite (R)-isomer did not perform as a teratogen [2]. At the time when thalidomide was sold, all medicinal drugs involving thalidomide were used as racemate because the technique of chiral chromatography and asymmetric synthesis was poorly developed and the importance of drug chirality was not well recognized. The drug disaster caused by thalidomide opened our eyes to the significance of chiral discrimination in drug development.
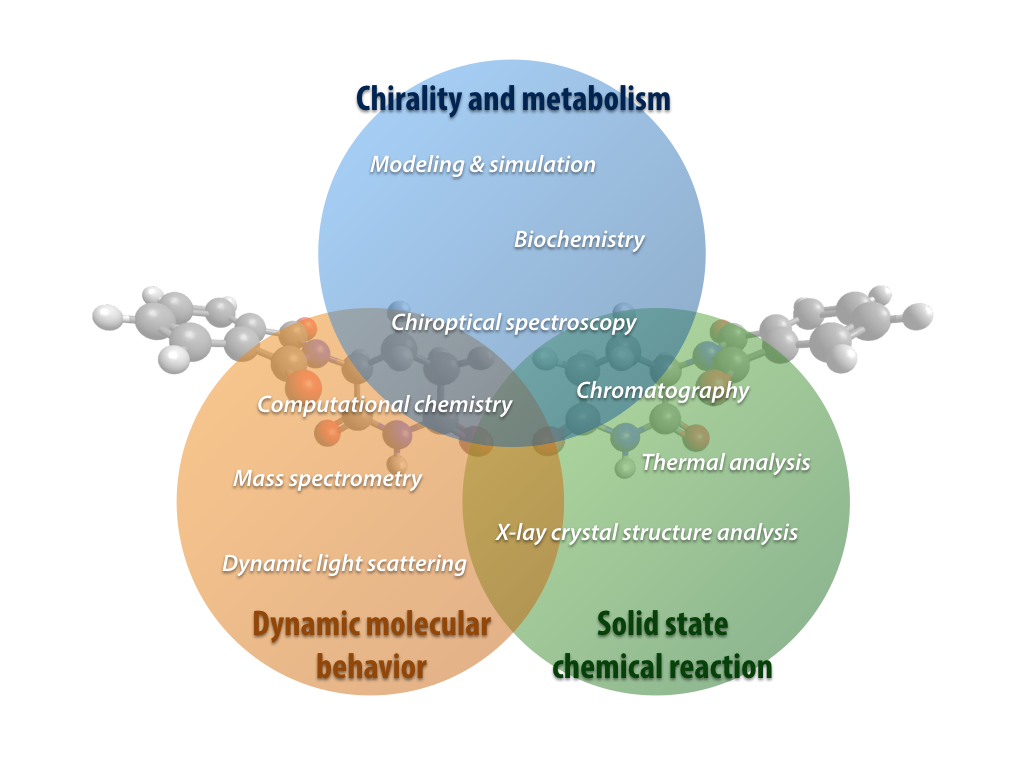
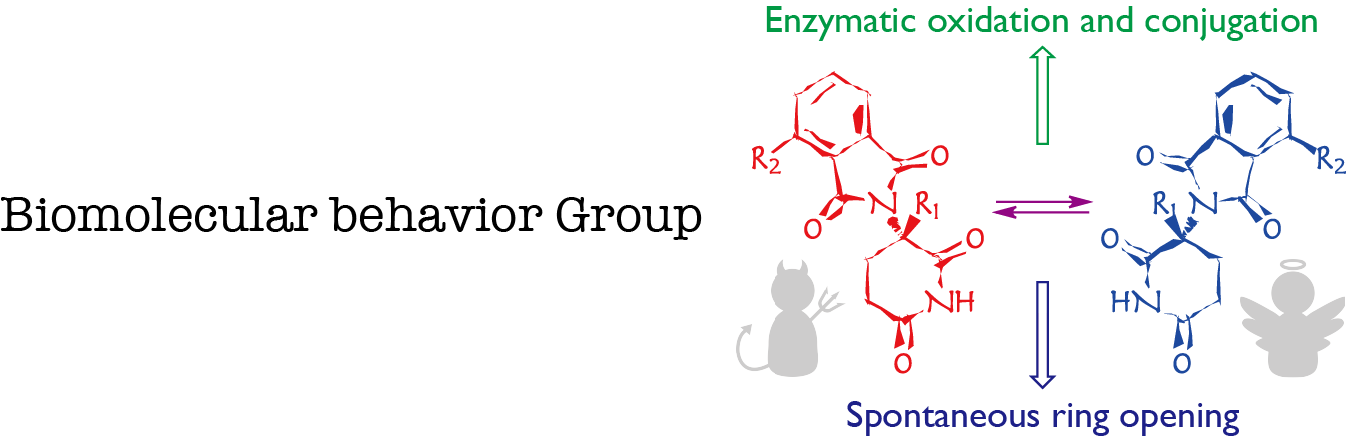
Figure 1. Our strategies for addressing thalidomide mechanism
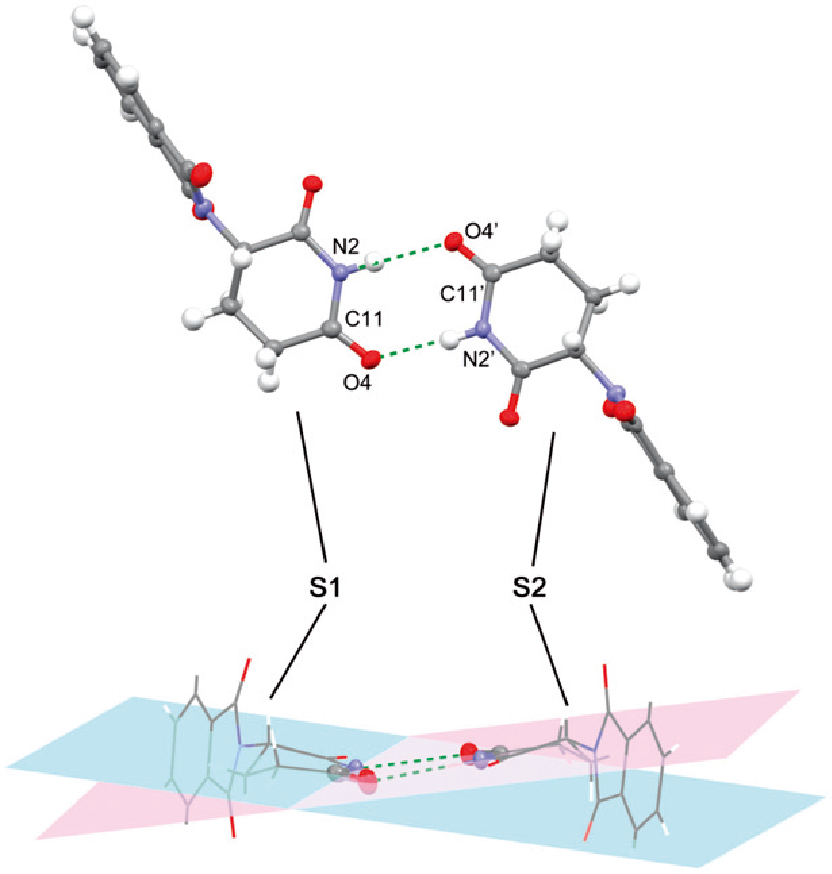
Right: Figure 2. Homo-chiral dimeric structure of thalidomide crystal (from ref.14)
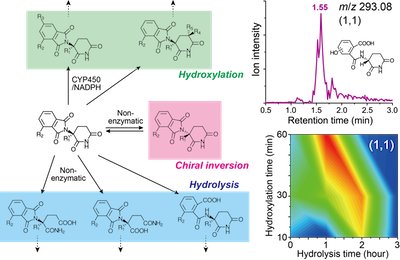
Figure 3. Quantification of thalidomide metabolites suffering from once hydrolysis and hydroxylation, and the metabolism map (from ref.17)
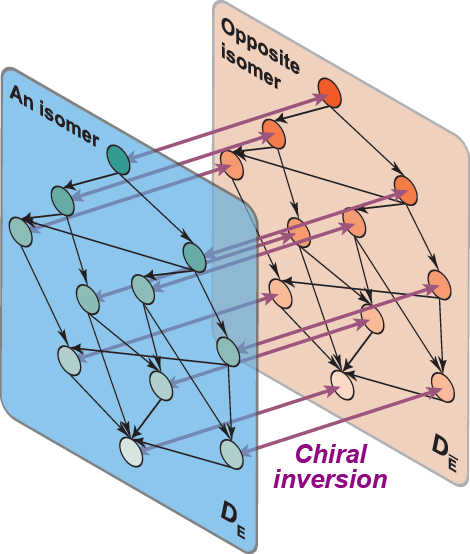
Right: Figure 4. Chemical reaction graph describing chiral inversion and metabolism (from ref.17)
Our group are also addressing the unique and interesting characteristics of thalidomide and other chiral molecules using experimental and theoretical approaches such as chiroptical spectroscopy, analytical chemistry, crystal optics, thermal analysis, quantum chemistry and mathematical modeling, like the following topics:
1) Experimental observation of simultaneous chiral inversion and biotransformation of thalidomide and its derivatives
2) Physicochemical properties of the metabolites of thalidomide and its derivatives
3) Dynamic multimer formation in thalidomide and its derivatives
4) Chemical reaction of thalidomide and its derivatives in solid state
References
[1] Lenz, W. et al. Lancet 1962, 279, 45-46. [2] Blaschke, G. et al. Arzneim. -Forsch. 1979, 29, 1640-1642.
[3] (a) Nishimura, K. et al. Chem. Pharmaceut. Bullet. 1994, 42, 1157-1159; (b) Eriksson, T. et al. J. Pharm. Pharmacol. 1998, 12, 1409-1416.
[4] Schumacher, H. et al. Brlt. J. Pharmacol. 1965, 25, 324-337. [5] Singhal, S. et al. N. Engl. J. Med. 1999, 341, 1565-1571.
[6] Barnhill, RL. et al. J. Am. Acad. Dermatol. 1982, 7, 317-323. [7] Hamuryudan, V. et al. Annals Int. Med. 1998, 128, 443-450.
[8] Wettstein, AR. et al. Lancet 1997, 350, 1445-1446. [9] Little, RF. et al. J. Clin. Oncol. 2000, 18, 2593-2602.
[10] (a) Takeuchi, Y. et al. Org. Lett. 1999, 1, 1571-1573; (b) Yamamoto, T. et al. Org. Lett. 2011, 13, 470-473.
[11] D'amato, RJ. et al. Proc. Natl. Aca. Sci. 2001, 28, 597-601. [12] Ito, T. et al. Science 2010, 327, 1345-1350.
[13] Fischer, ES. et al. Nature 2014, 512, 49–53. [14] Suzuki, T. et al. Phase Transition 2010, 83, 223-234.
[15] Soloshonok, VA. Angew. Chem. Int. Ed. 2006, 45, 766-769. [16] Maeno, M. et al. Chem. Sci. 2015, 6, 1043-1048.
[17] Ogino Y. et al. Chirality 2017, in press. [18] Ogino, Y. and Asahi, T. J. Theo. Biol. 2015, 373, 117-131.
